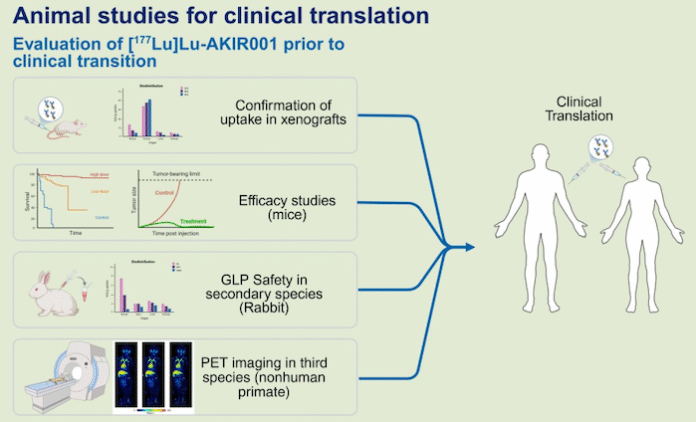
Akiram Therapeutics, a Swedish biotech company specializing in targeted radiotherapy, has announced that data on its CD44v6-targeted radiotherapeutic candidate AKIR001 have been published in The Journal of Nuclear Medicine, one of the leading journals in nuclear medicine.
The publication summarizes key studies underpinning the ongoing first-in-human Phase 1 trial at Karolinska University Hospital. It reports high tumor selectivity, favorable safety and dosimetry findings, and antitumor effects in multiple preclinical models.
The article, titled ‘Preclinical Validation of [177Lu]Lu-AKIR001, a CD44v6-Targeted Radiotherapeutic Entering First-in-Human Trials,’ presents foundational data supporting the clinical development of AKIR001.
The study shows that the candidate exhibits a favorable tumor-to-organ profile, with sustained retention in tumors and low uptake in normal tissue — key characteristics of radiopharmaceuticals. The combined findings from biodistribution, dosimetry and specificity analyses confirm that 177Lu-AKIR001 demonstrates clear and selective targeting of CD44v6-expressing tumors.
The efficacy and safety results further strengthen the rationale for clinical evaluation, with clear dose-dependent antitumor activity and low uptake in normal tissues across several preclinical systems. These findings provide important scientific validation for Akiram’s CD44v6-targeted approach and support the ongoing first-in-human trial in patients with tumors that express CD44v6.
“These results bring together years of systematic development around our CD44v6 platform—spanning antibody engineering, dosimetry, in vivo studies and safety assessments. Having the work peer-reviewed adds an extra layer of confidence as the drug is now being evaluated in patients at Karolinska University Hospital. For us, it reaffirms that we are advancing on a solid and well-supported scientific foundation,” says Marika Nestor, CEO of Akiram Therapeutics.
The ongoing first-in-human Phase 1 trial is conducted and sponsored by Karolinska University Hospital. The study evaluates safety, tolerability, pharmacokinetics and biodistribution in patients with tumors that express CD44v6, including anaplastic and iodine-refractory thyroid cancer, head and neck squamous cell carcinoma, gynecological squamous cell carcinoma and non-small cell lung cancer.