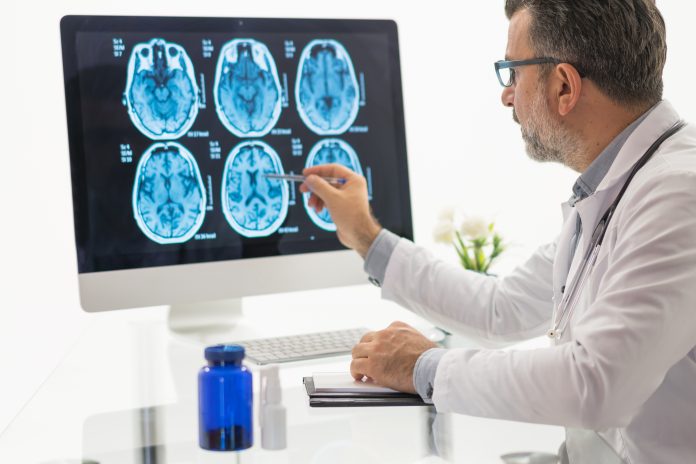
Pierre Fabre Laboratories recently announced the filing of an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) to initiate a first-in-human (FIH) Phase I/II clinical trial with PFL-002/VERT-002 for solid tumors including non-small cell lung cancer (NSCLC).
The PFL-002/VERT-002 Phase I/II trial is a multi-center, international study aimed at assessing the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary efficacy of PFL-002/VERT-002 in NSCLC patients with MET alterations, including those acquired as a resistance mechanism to other treatments. The FDA will review the application and determine its acceptability.
“We are looking forward to initiating the first-in-human trial of PFL-002/VERT-002 later this year. We are confident that this new drug holds significant promise, as a novel therapeutic option with a differentiated mechanism of action, for patients facing MET-altered solid tumors, including NSCLC” said Francesco Hofmann, Head of Research and Development for Medical Care at Pierre Fabre Laboratories.