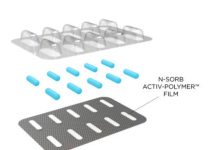
Nipro PharmaPackaging and Nemera have announced the successful compatibility testing of Nemera’s UniSpray and Nipro’s unit-dose microvials. This collaboration aims to accelerate the time to market for combination products, particularly those used for rescue and crisis intervention.
Nemera’s UniSpray, a single-metered dose device for systemic drug administration, has been rigorously tested and proven fully compatible with Nipro’s unit-dose microvials. The comprehensive test program and assessment confirmed the combination product’s reliability, safety, and performance, ensuring that UniSpray meets the required dose delivery and reliability standards thanks to its robust design.
“At Nemera, we design and manufacture devices that maximize treatment efficacy,” said Séverine Duband, Nemera’s Marketing Director for Devices. “We partner with our customers in the best possible way to ultimately bring patients high-quality and safe treatment solutions. Our work with primary drug packaging partners like Nipro further strengthens our commitment to bringing innovative combination products to customers and patients.”
Clément Condouret, NPI product portfolio manager for Nipro, said, “The high dimensional and cosmetic quality of Nipro unit-dose vials is crucial for ensuring optimal compatibility with Nemera’s UniSpray. This precision enhances the drug delivery system’s reliability, safety, and performance, ultimately contributing to the efficacy and safety of patient treatments.”