Global pharmaceutical company Dr. Reddy’s Laboratories has announced the launch of the Nerivio Remote Electrical Neuromodulation (REN) wearable through its subsidiary betapharm. Nerivio is a United States Food and Drug Administration (USFDA), and CE-marked device that uses remote electrical neuromodulation (REN) to prevent and treat migraine. The novel wearable device is a drug-free treatment made by Theranica, a neuromodulation therapeutics company based in Israel and the US.
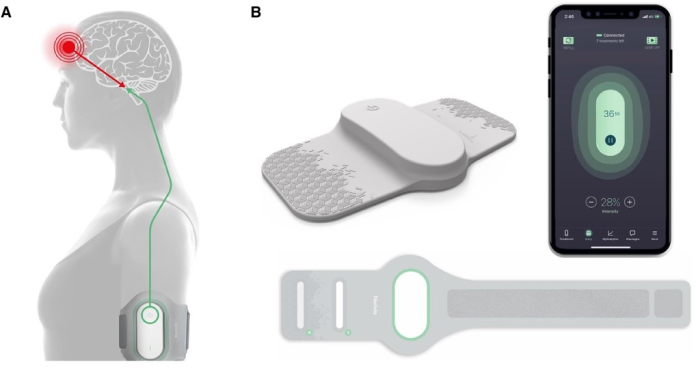
Nerivio is a prescription-based, non-invasive device intended for acute and prophylactic (preventive) treatment of migraine with or without aura for adults and adolescents aged 12 years and older. Nerivio can be worn on the upper arm and controlled by a smartphone app. Each device has an in-built capability for providing 18 treatment sessions, each 45 minutes long.
It is to be used within 60 minutes of the onset of headache or aura for acute treatment of migraine or every alternate day for prevention of migraine. The device uses the Remote Electrical Neuromodulation (REN) mechanism to activate conditioned pain modulation, specifically by stimulating nociceptive neural receptors under the skin of the arm. This initiates a natural pain-relieving process in the brainstem, causing a global effect of pain inhibition that affects the source of migraine pain in the brain.
“Neuromodulation offers a significant advancement in migraine treatment,” says Dr. Arne May, a leading researcher in the department of Systems Neuroscience at the University of Hamburg and Head of the headache outpatient clinic of the University Clinic of Hamburg. “For decades, medication has been the mainstay of migraine management. However, many patients experience side effects or limited efficacy with these medications. Neuromodulation technologies offer a much-needed alternative. Such a modern neuromodulation technology is Nerivo. By stimulating specific neural pathways, Nerivio can trigger the brain’s natural pain-relieving mechanisms, which were found to be deficient among migraine patients, without introducing chemical substances into the system. This drug-free approach holds particular promise for vulnerable populations such as adolescents and women of childbearing age, who may not be able to take conventional migraine medications.”
Patrick Aghanian, chief executive officer of Dr. Reddy’s Europe, commented, “Starting with Germany, we are excited to introduce Nerivio in Europe as a new drug-free innovation that can help migraine patients. Remote Electrical Neuromodulation (REN) has the potential to reduce the number of pills patients need to take and decrease their dependence on non-specific medication for chronic or hard-to-treat diseases. Worn on the upper arm and controlled by a smartphone app, it is comfortable, discreet and portable. We believe that gives patients the freedom to focus on their daily activities while wearing it, addressing the unmet clinical need for more convenient migraine treatment, which significantly impacts well-being and quality of life.”
Clinically proven in multiple clinical trials6, the drug-free REN wearable is suitable for a wide range of migraine patients, especially those who seek an individual treatment opportunity, a drug-free therapy, failed to control their migraine with medications, or with poor tolerance or contraindication to medications. In addition, sensitive populations such as adolescents, women of childbearing age groups, and patients at risk of medication overuse headaches could benefit from Nerivio.
Studies show that Nerivio is well-tolerated with no systemic side effects or concern for medication overuse. It has been evaluated in robustly designed pivotal studies conducted in the US and Israel in patients seeking effective acute or preventive treatment of migraine. Nerivio has proven efficacy in not only effectively providing relief from pain but also associated migraine symptoms such as nausea or vomiting, phonophobia, and photophobia. It has been shown to reduce the need for drugs used for abortive treatment.
In August 2023, Nerivio received an expanded CE mark approval under MDR European regulation as a dual-use (acute and/or prevention) migraine treatment for adolescents and adults. The launch in Germany is the result of multiple exclusive agreements signed between Dr. Reddy’s European subsidiaries and Theranica for the commercial marketing and distribution of Nerivio in Germany, Austria, Czech Republic, Denmark, Finland, France, Italy, Norway, Poland, Slovakia, Spain, Sweden, Switzerland, and the United Kingdom. These markets will likely follow with planned launches in Spain and the UK between May and June.
The product has already been presented in Germany at the DGN Kongress 2023, organized by the German Association of Neurology in Berlin, following a successful launch in India in November 2023.
The prescribed Nerivio REN wearable is indicated for acute and/or preventive treatment of migraine with or without aura in patients 12 years and older. Patients are advised to consult their physician or neurologists on the use of the device and management of migraine.