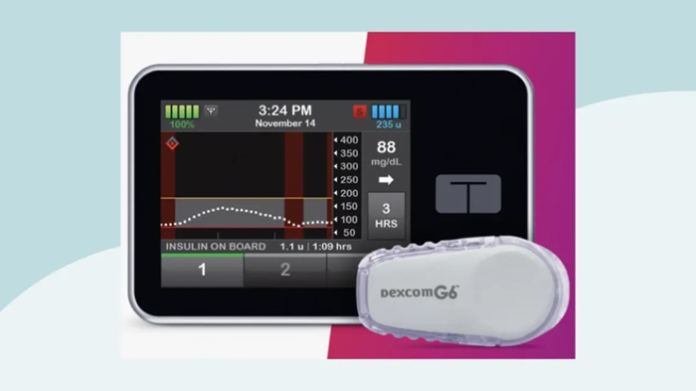
Abbott, a global health care company, and Tandem Diabetes Care, a leading insulin delivery and diabetes technology company, has announced that the t:slim X2 insulin pump with Control-IQ technology is the first automated insulin delivery (AID) system to integrate with the newly available FreeStyle Libre 2 Plus sensor, Abbott’s latest continuous glucose monitoring (CGM) technology.
For the first time, FreeStyle Libre technology users in the United States are now able to experience the therapeutic benefits of a hybrid closed-loop system that helps predict and prevent high and low blood sugar.
Tandem’s t:slim X2 insulin pump connects wirelessly to the FreeStyle Libre 2 Plus sensor, which sends automatic glucose readings every minute2,3 to the pump. Users can conveniently see their minute-to-minute glucose data on the pump and accompanying t:connect mobile app.
The pump’s Control-IQ technology predicts glucose levels 30 minutes into the future, automatically adjusting insulin delivery every five minutes based on CGM readings and can deliver automatic correction boluses (up to one per hour) to help prevent hyperglycemia5. When left untreated, hyperglycemia can lead to long-term diabetes complications, such as nerve and kidney damage or disease of the eyes.
“Tandem’s leadership in AID innovation is underscored with this milestone of launching the first insulin pump to be compatible with Abbott’s CGM technology in the US,” said John Sheridan, president and chief executive officer of Tandem Diabetes Care. “This represents another step forward in our commitment to provide customizable solutions to help reduce burden and create new possibilities for people living with diabetes.”
Abbott’s latest addition to the FreeStyle Libre portfolio is the FreeStyle Libre 2 Plus sensor, which is the modified FreeStyle Libre 2 sensor cleared in 2023 by the U.S. Food & Drug Administration for use with AID systems. It is the first and only CGM available in the United States with a wear time of 15 days1 for both adults and children. Demonstrating proven accuracy in overall glucose readings with an 8.2% MARD6, the sensor is small, discreet7 and comfortable8 to wear.
“Our FreeStyle Libre portfolio empowers people living with diabetes to have more control over their health and eases the burden of their condition by using a continuous glucose monitoring system with unsurpassed accuracy6 that is simple, easy and affordable to use,” said Jared Watkin, executive vice president of Abbott’s diabetes care business. “Through the integration of Tandem’s t:slim X2 insulin pump with our FreeStyle Libre 2 Plus sensor, people only need two sensors a month, which ensures both a more convenient and more affordable experience.”
Tandem will email instructions to all in-warranty t:slim X2 users in the U.S. offering the option to add the new FreeStyle Libre 2 Plus sensor integration at no cost via remote software update. t:slim X2 pumps pre-loaded with the updated software are now shipping to new customers. Information about the software update process for existing in-warranty pump users, including system requirements, is available at tandemdiabetes.com/softwareupdate.
The FreeStyle Libre 2 Plus sensor is available now at participating durable medical equipment (DME) suppliers.
GlobalData insights
This announcement provides another useful treatment option for diabetic patients requiring insulin therapy and who wish to utilize continuous glucose monitoring, says GlobalData, a leading data and analytics company.
GlobalData forecasts that the diabetes market in the US is expected to grow at a compound annual growth rate (CAGR) of 5.35% between 2021 and 2025, increasing to approximately $11.6 billion by 2025. According to the GlobalData Marketed Products Database, Abbott and Tandem Diabetes Care have a combined 45 devices currently on the diabetes market, of which 22 are continuous glucose monitors or insulin pumps.
David Beauchamp, medical analyst at GlobalData, comments: “Growth in the diabetes market is precipitated by new diagnoses and technologies developed by companies such as Abbott and Tandem Diabetes Care. Major growth areas include insulin delivery and glucose monitoring systems, which both companies provide to diabetic patients. The integration of their devices can lead to greater control over blood glucose levels and prevent complications arising from high or low blood glucose levels.”
Ensuring proper management of blood glucose levels is a vital component of diabetes care, especially for those requiring insulin therapy. Insulin pumps as a standalone treatment can already lead to better glucose control through more exact measurement and easy-to-administer insulin, and integrating continuous glucose monitors such as the FreeStyle Libre 2 Plus promises to further enhance patients’ ability to control their blood glucose levels through 24/7 monitoring and early warning of high or low blood glucose levels.
Beauchamp concludes: “The use of insulin pumps and continuous glucose monitors together provides a closed-loop solution for insulin therapy that promises to significantly improve blood glucose control in patients. Ensuring future compatibility between the various insulin pumps and continuous glucose monitors currently on the market will provide additional options for diabetic patients to better manage their blood glucose levels and prevent complications from poor glucose control.”