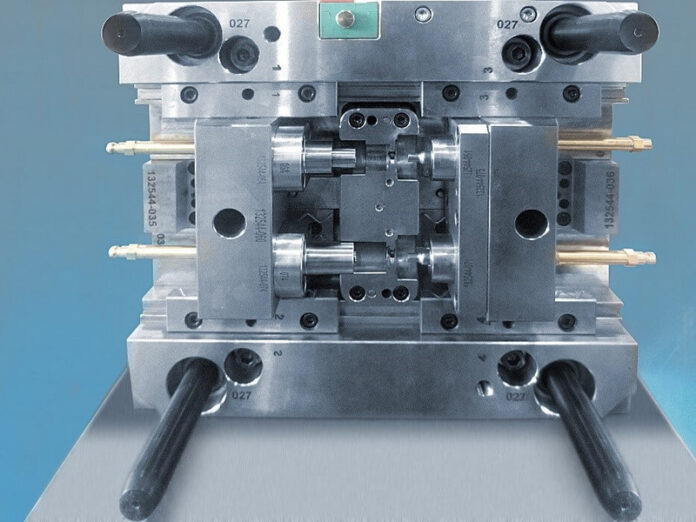
To date, injection molding tools have been maintained and optimized at the Gerresheimer location in Dongguan (China). Since certification, according to DIN ISO 9001 in July 2020, the company now also builds complete molds. Gerresheimer, in this way, makes itself more independent of local mold makers and can offer the professional, cost-efficient construction of injection molding tools for its customers extending beyond its own needs. A team of eight employees produces mold prototypes for its customers at the Dongguan location.
The maintenance and optimization of molds are still part of the service offering. In addition to this, molds for series production in Asia, Europe, and the USA are manufactured there. “With the expanded portfolio of offerings in mold making, we adjust to the needs of our various customer segments. In the pharmaceuticals and medical technology markets, cost-efficient molds that can be delivered quickly for start-ups and development projects are required. We now offer the appropriate solution for precisely this,” explains Manfred Baumann, global executive vice president Sales, and Marketing, Administration and TCC, Gerresheimer Medical Systems.
Mold making in Dongguan consciously understands itself not as competition, but instead as supplementing the more comprehensive offering of services at the German location of Wackersdorf. While highly complex, high-cavity molds can be built for pharmaceutical and medical technology large series production, the offering of services in China is limited to molds with an average degree of complexity. What distinguishes mold making in Dongguan much more is German quality standards with the speed and the attractive prices of the China location.
Gerresheimer already started in 2018 by redesigning its department for mold maintenance and optimization at the South Chinese production location in Dongguan into a full-fledged mold making facility. In 2019, a start was made toward creating the process description, which is the prerequisite for certification according to the quality management standard DIN ISO 9001. The certification audit then took place in May 2020 – for the first time in an online process due to the corona pandemic. The quality management system of the location is now certified by the DQS, taking effect on July 19, 2020.
Gerresheimer is a leading international partner of the pharmaceuticals and health care industries. The company contributes to health and well-being with our special products of glass and plastics. Gerresheimer is represented worldwide and, with around 10,000 employees, produces where its customers and markets are. With works in Europe, North and South America, and Asia, Gerresheimer achieves a turnover of around EUR 1.4 billion. The broad range of the offering encompasses pharmaceutical packaging, as well as products for the simple and secure administering of medications such as insulin pens, inhalers, pre-fillable syringes, injection vials, ampules, bottles, and containers for liquid and solid medications with sealing and safety systems, as well as packaging for the cosmetics industry.







