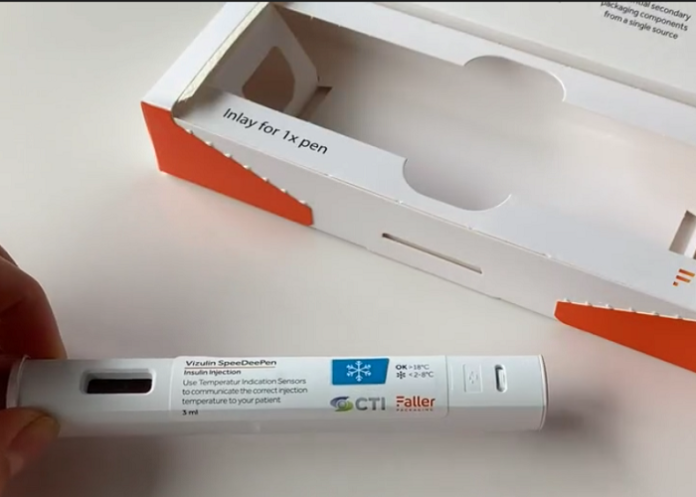
The trend toward medicines that are self-administered at home is creating new challenges in the pharmaceutical industry. For example, how can the task of administering their own medications be made easier for patients? Germany-based August Faller Packaging has an to this question – labels with printable indicators for temperature and light.
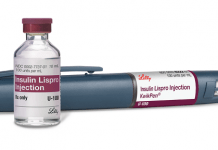
Self-application medicines should be as simple and safe to use as possible which helps in achieving therapy compliance. Incorrectly administered medications can have unpleasant consequences for patients. For example, insulin can cause pain if it is too cool – and in the worst-case scenario, the insulin’s altered viscosity can even delay its effectiveness.

Photo August Faller
To remedy this and further minimize the cognitive burden for the patient, Faller and its partner CTI (Chromatic Technologies) have developed labels with printable temperature or light indicators. Users at home know they should administer the medication when the label indicator changes color. The label’s design and required indication area can be individually adapted to pharmacists’ requirements. The shape, color, temperature range, branding, text, and other features can be personalized on request. The sensor is reversible or irreversible depending on the desired usage scenario.
Product Marketing manager at Faller Packaging Sarina Diebold who has closely followed the development of the new labels and their market launch, says, “The customer can easily print the indicator on the label without the need for electronics. Our smart solution replaces expensive technologies that are often bulky and rigid and protrude from the packaging. So like any other common label, the new label can be further processed downstream and simply applied to the product.”
The product innovation process (PIP) is driven by an agile stage-gate process that’s optimally tailored. The innovation process for the new labels began about two years ago. During this period, with an intensive market focus, the innovation was tested for relevance and feasibility with the target group. Direct interaction with potential pharmaceutical companies at a very early stage also played an important role in its development and optimization for users.
There is a great deal of interest in the innovative labels and August Faller is discussing potential usage scenarios with several customers. Individual products will be developed together with customers and its partner CTI. However, it’s important to understand exactly what the indicator on this label actually represents.