Royal Philips, a global leader in health technology, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new Liver Fat Quantification tools as part of the latest release of its ultrasound systems EPIQ Elite and Affiniti, bringing the cost and accessibility advantages of sonography to the diagnosis of early-stage liver disease. Featured at this year’s Radiological Society of North America (RSNA) annual meeting 23 November – 2 December, Chicago, USA, the new tools will allow clinicians to track liver health.
Fatty liver disease is the most common and earliest stage of chronic liver disease. The incidence of non-alcoholic fatty liver disease (NAFLD) is increased by various risk factors, including Type 2 diabetes and obesity. It is estimated that NAFLD may be present in about 25% of the global population. NAFLD includes milder fatty liver disease, as well as more severe forms that include inflammation, a condition called Non-Alcoholic Steatohepatitis (NASH), and fibrosis. Research suggests that early intervention enables patients to adopt lifestyle changes that can help to prevent liver disease, while early assessment of fatty liver is key to potentially reversing this progression of liver disease.
With a quantitative way of measuring liver fat, it is a lot easier for us to let the referring physician know where the patient is quantitatively on the NAFLD spectrum, quoted Richard G. Barr, MD, PhD, president, Radiology Consultant, Youngstown, Ohio, U.S.A., and Medical director at Southwoods Imaging.
With the extended remote functionality of Collaboration Live on both the EPIQ and Affiniti platforms, technicians can also securely access on-demand, real-time guidance and decision support to enhance diagnostic confidence and workflow efficiency during exams.
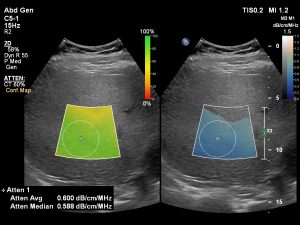
photo Philips
“Today’s announcement demonstrates the continued advancement of our ultrasound portfolio to increase diagnostic confidence and workflow efficiency,” said Jeff Cohen, general manager of Ultrasound at Philips. “Accessible ultrasound-based Liver Fat Quantification is a screening and early diagnostic tool that will allow many more patients to take their health into their own hands by making simple lifestyle changes.”
Enhancing the diagnosis and treatment of early-stage and advanced liver disease
Philips will debut its Liver Fat Quantification solution at the Radiological Society of North America (RSNA) Annual Meeting later this month. For more information on Philips Liver Fat Quantification, including live demos, where the company will spotlight its latest portfolio of radiology workflow solutions and smart connected imaging systems to increase efficiency and diagnostic confidence in precision care and treatment.