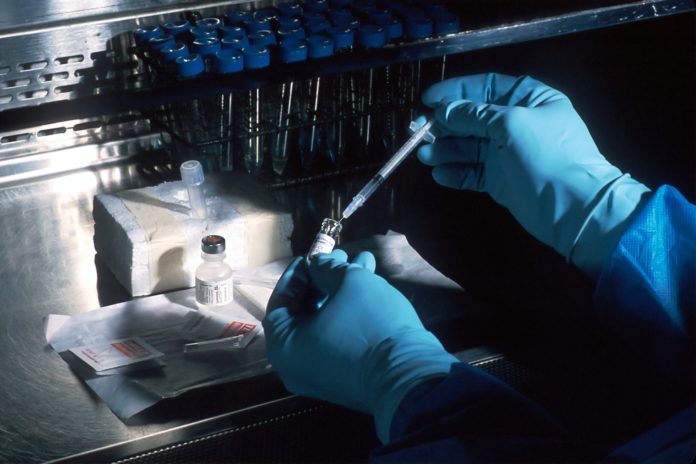
Sanofi and GSK are in advanced discussions with the European Commission (EC) for the supply of up to 300 million doses of a Covid-19 vaccine. The vaccine candidate developed by Sanofi in partnership with GSK is based on the recombinant protein-based technology used by Sanofi to produce an influenza vaccine, and GSK’s established adjuvant technology. The doses would be manufactured in European countries, including France, Belgium, Germany and Italy. This marks a key milestone in protecting and serving the European population against Covid-19.
“Today’s announcement helps ensure that millions of Europeans will have access to a potential vaccine protecting against Covid-19, once proven safe and effective. Our steadfast commitment to providing a vaccine that is affordable and accessible to everyone, and we are grateful to the European Commission for their ongoing engagement and shared support of this effort,” said Thomas Triomphe, executive vice president and global head of Sanofi Pasteur. “Together with GSK, we are working relentlessly to develop and produce a vaccine to address this global health crisis.”
Roger Connor, president of GSK Vaccines, added, “GSK is proud to be working in partnership with Sanofi to make this vaccine available as soon as possible in Europe. Both companies have significant R&D and manufacturing capability in Europe and are already working hard to scale up production across our networks. This announcement from the EC supports our ongoing efforts.”
Sanofi is leading the clinical development and registration of the Covid-19 vaccine and expects a Phase 1 and 2 study to start in September, followed by a Phase 3 study by the end of 2020. If data are positive, regulatory approval could be achieved by the first half of 2021. In parallel, Sanofi and GSK are scaling up manufacturing of the antigen and adjuvant to produce up to one billion doses per year overall.
Sanofi and GSK recently signed agreements with the United States, where they have long-standing partnerships with the Biomedical Advanced Research and Development Authority and the UK Government. The partners plan to provide a significant portion of total worldwide available supply capacity in 2021/22 to the global initiative “Access to Covid‐19 Tools (ACT) Accelerator,” a global collaboration of leaders of governments, global health organizations, businesses and philanthropies to accelerate development, production, and equitable access to Covid-19 tests, treatments, and vaccines.
In addition to the recombinant protein-based vaccine in collaboration with GSK, Sanofi is also developing a messenger RNA vaccine candidate in partnership with Translate Bio. With several innovative vaccine platforms currently investigated across the industry, mRNA is considered among the most promising. Sanofi expects a Phase 1 study to start by the end of the year, and, if data are positive, approval at the earliest in the second half of 2021. Translate Bio has established mRNA manufacturing capacity and Sanofi expects to supply an annual capacity of 90 to 360 million doses.